100763
论文已发表
提 交 论 文
注册即可获取Ebpay生命的最新动态
注 册
IF 收录期刊
- 3.3 Breast Cancer (Dove Med Press)
- 3.4 Clin Epidemiol
- 2.5 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.5 Clin Interv Aging
- 4.7 Drug Des Dev Ther
- 2.7 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.5 Int J Women's Health
- 2.5 Neuropsych Dis Treat
- 2.7 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.3 Ther Clin Risk Manag
- 2.5 J Pain Res
- 2.8 Diabet Metab Synd Ob
- 2.8 Psychol Res Behav Ma
- 3.0 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.7 Risk Manag Healthc Policy
- 4.2 J Inflamm Res
- 2.1 Int J Gen Med
- 4.2 J Hepatocell Carcinoma
- 3.7 J Asthma Allergy
- 1.9 Clin Cosmet Investig Dermatol
- 2.7 J Multidiscip Healthc

阿帕替尼 (Apatinib) 用于 EGFR 野生型和 ALK 阴性晚期肺腺癌的后二线治疗
Authors Fang S, Zhang H, Zhang Y, Xie W
Received 4 November 2016
Accepted for publication 8 December 2016
Published 18 January 2017 Volume 2017:10 Pages 447—452
DOI http://doi.org/10.2147/OTT.S126613
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Ru Chen
Peer reviewer comments 3
Editor who approved publication: Dr Yao Dai
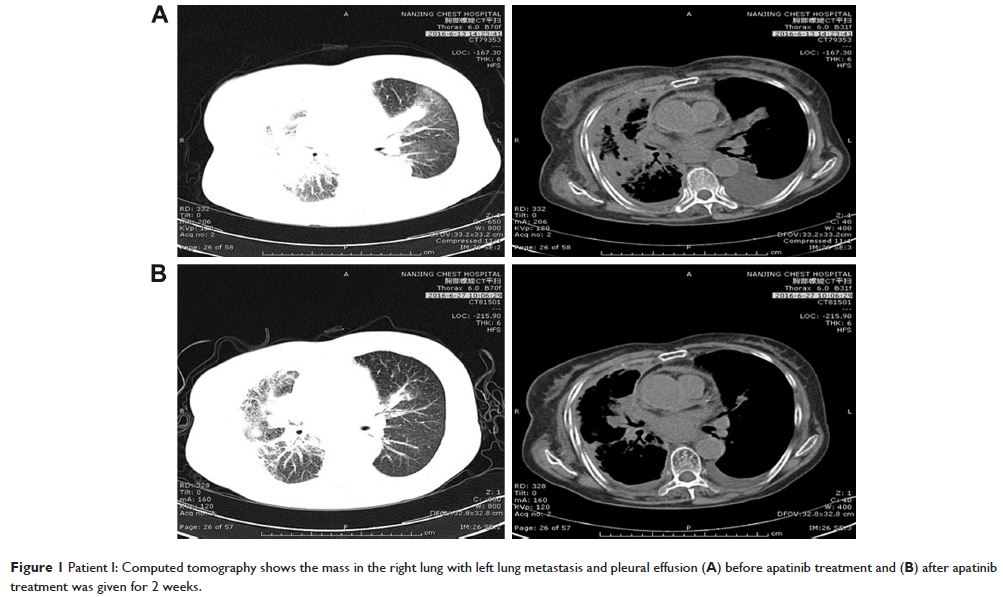
Abstract: In the absence of a driver mutation, chemotherapy is the standard
treatment option as first- and second-line therapy for advanced non-small-cell
lung cancer (NSCLC). Though a large number of patients are suitable for post
second-line therapies, the quality and quantity of the available drugs in this
setting is poor. Apatinib, a small molecule vascular endothelial growth factor
receptor-2 (VEGFR-2) tyrosine kinase inhibitor, is a first-generation oral
antiangiogenesis drug approved in the People’s Republic of China for use as a
subsequent line of treatment for advanced gastric cancer. Herein, we report
three cases of advanced NSCLC with epidermal growth factor receptor wild-type
and anaplastic lymphoma kinase-negative status, wherein the patients showed
partial response to apatinib. Moreover, the three patients have achieved a
progression-free survival of 2.8, 5.8, and 6 months, respectively. The main
toxicities were hypertension, proteinuria, and hand–foot syndrome. Apatinib may
provide an additional option for the treatment of advanced NSCLC, especially
for advanced lung adenocarcinoma without a driver mutation.
Keywords: non-small-cell
lung cancer, angiogenesis, apatinib, VEGFR-2